3.0 Chemical Bonding
NOTE: It may appear that there are gaps on your phone, ensure you close each table section for a better scrolling experience!
Candidates should be able to:
(a) show understanding that all chemical bonds are electrostatic in nature and describe:
(i) ionic bond as the electrostatic attraction between oppositely charged ions
(ii) covalent bond as the electrostatic attraction between a shared pair of electrons and positively charged nuclei
(iii) metallic bond as the electrostatic attraction between a lattice of positive ions and delocalised electrons
(b) describe, including the use of ‘dot-and-cross’ diagrams,
(i) ionic bonding as in sodium chloride and magnesium oxide
(ii) covalent bonding as in hydrogen; oxygen; nitrogen; chlorine; hydrogen chloride; carbon dioxide; methane; ethene
(iii) co-ordinate (dative covalent) bonding, as in formation of the ammonium ion and in the Al2Cl6 molecule (c) describe covalent bonding in terms of orbital overlap (limited to s and p orbitals only), giving σ and π bonds (see also Section 11.1)
(d) explain the shapes of, and bond angles in, molecules such as BF3 (trigonal planar); CO2 (linear); CH4 (tetrahedral); NH3 (trigonal pyramidal); H2O (bent); SF6 (octahedral) by using the Valence Shell Electron Pair Repulsion theory
(e) predict the shapes of, and bond angles in, molecules analogous to those specified in (d)
(f) explain and deduce bond polarity using the concept of electronegativity [quantitative treatment of electronegativity is not required]
(g) deduce the polarity of a molecule using bond polarity and its molecular shape (analogous to those specified in (d));
(h) describe the following forces of attraction (electrostatic in nature): (i) intermolecular forces, based on permanent and induced dipoles, as in CHCl3(l); Br2(l) and the liquid noble gases (ii) hydrogen bonding, using ammonia and water as examples of molecules containing –NH and –OH groups
(i) outline the importance of hydrogen bonding to the physical properties of substances, including ice and water
(j) explain the terms bond energy and bond length for covalent bonds
(k) compare the reactivities of covalent bonds in terms of bond energy, bond length and bond polarity
(l) describe, in simple terms, the lattice structure of a crystalline solid which is: (i) ionic, as in sodium chloride and magnesium oxide (ii) simple molecular, as in iodine (iii) giant molecular, as in graphite and diamond (iv) hydrogen-bonded, as in ice (v) metallic, as in copper [the concept of the ‘unit cell’ is not required]
(m) describe, interpret and/or predict the effect of different types of structure and bonding on the physical properties of substances
(n) suggest the type of structure and bonding present in a substance from given information
Taken from Chemistry Singapore-Cambridge General Certificate of Education Advanced Level Higher 2 (2019) Syllabus
Fundamental Ideas
-
A chemical bond refers to the attractive forces between atoms, ions or molecules that result in a lower energy arrangement.
-
Inter-atomic bonds refer to attractive forces between atoms or ions.
-
Inter-molecular forces of attraction are attractive forces between atoms or molecules.
3.1 Ionic Bonding
-
An ionic bond is the strong electrostatic forces of attraction between oppositely charged ions.
-
Ions in ionic compounds are formed due to electron transfer from usually a metallic atom to a non-metallic atom.
-
The ions have a stable and complete octet structure.
The following example is a classic example of ionic bonding in sodium chloride found in salt. Each sodium atom loses its valence electron which forms a stable Na+ cation. This electron is gained a Cl atom to form a Cl- ion which also has a stable configuration.
3.1.1 Ionic Bond Strength
-
Ionic bonds are strong and non-directional (equally strong in all directions). A lot of energy is required to break this bond.
-
However, it is also dependent on two factors, charge and ionic radius which gives rise to differing melting points of different ionic compounds, e.g MgO and NaCl.
-
Lattice energy is the amount of energy evolved when one mole of ionic compound is formed from its constituent gaseous ions. The higher the LE, the stronger the ionic bond.
-
L.E ∝ (q+ * q-)/ (r+ + r-)
-
Larger ionic charge results in higher LE, smaller ionic radius results in higher LE.
3.1.2 Properties of Ionic Compounds
-
High melting/boiling point: Strong electrostatic forces of attraction hold oppositely charged ions in fixed positions, which require a high amount of energy to break and hence a high melting/boiling point.
-
Hard and brittle: Ionic bonds are strong and hold ions in fixed positions. Dislocation of ions brings like charges close resulting in strong repulsion (like charges repel) causing fracture.
-
Soluble in polar solvents: Ions have a strong charge dipole thus forming strong ion-dipole interactions with polar molecules which release a large amount of energy that could break the ionic bonds holding the ions together allowing it to dissolve.
-
Conduct electricity in molten or aqueous states only: Ions are held in fixed positions by strong ionic bonds in solid state, thus creating absence of charge carriers preventing the compound from conducting electricity. The opposite happens when molten or dissolved as the compound has dissolved.
3.2 Metallic Bonding
-
Metallic bonding is found in metals.
-
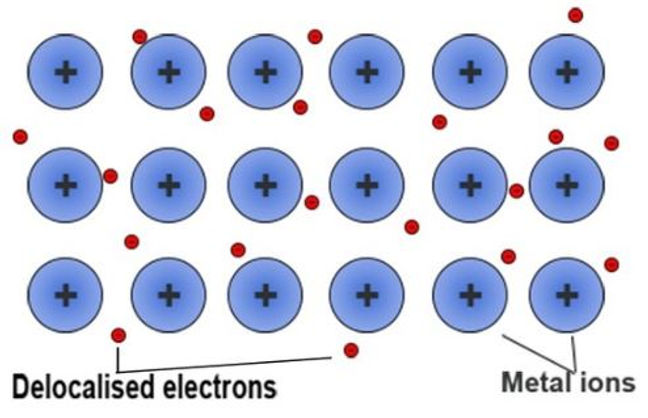
Metallic bonding is the strong electrostatic forces of attraction between the metal ions and the sea of delocalised electrons.
-
Metals consist of cations in regular layers and it being covered by a sea of electrons.
3.2.1 Factors affecting Metallic Bonds
In order of priority,
-
More the delocalised electrons per cation, the stronger the metallic bond.
-
Higher the charge density, stronger the metallic bond.
3.2.2 Properties of Metals
-
High melting point: Strong electrostatic forces of attraction between cations and sea of electrons require a large amount of energy to overcome.
-
High density due to close packing of cations.
-
Malleable as cations are arranged in orderly layers allowing the layers to slide over one another with a single force. The delocalised electrons move to prevent repulsion between cations
-
Ductile: Able to be pulled into wires
-
Insoluble in both polar and non-polar solvents: Energy evolved from ion-dipole interaction are not enough to overcome strong metallic bonds.
3.3 Covalent Bonding
-
Covalent bond is the strong electrostatic forces of attraction between the nuclei of each atom and a shared pair of electrons.
-
Covalent bonds are formed when the orbitals of two neighboring atoms overlap. The overlap can be head-on or side-on.
-
Head-on overlap forms a sigma bond. A side-on overlap forms a pi bond. First covalent bond between atoms is sigma then pi bonds if more bonds are to be formed between the same atoms, i.e. a double bond consists of one sigma, one pi.
-
A pi bond is weaker than sigma bond due to decreasing extent of overlap.
3.3.1 Strength of Covalent Bond
-
Triple Bond> Double Bond> Single Bond in terms of strength.
-
Bond Length: Further the nuclei, weaker the bond.
-
Bond Polarity: If two atoms are of differing electronegativity which results in polarization of bonding atoms, there will be an ionic character and hence a stronger attraction between the atoms making the covalent bond stronger.
-
Lone Pair Proximity: If lone pair of bonding atoms are close, it may result in extra repulsion weakening the covalent bond.
3.3.2 Representation of Covalent Bonding
-
In covalent bonding, electrons are shared so that each atom will acquire noble gas configuration.
-
Lone pairs are the outermost shell electron pairs which are not involved in bonding.
-
Bond pairs are the electron pairs involved in bonding.
-
Elements in Period 3 and beyond can expand their octet due to the availability of energetically and accessible d orbitals.
3.3.3 Properties of Covalent Compounds
Simple Covalent Molecules
-
Low melting/boiling points: Weaker intermolecular forces of attraction arise between molecules which require a small amount of energy to overcome.
-
Insoluble in polar but soluble in non-polar solvents.
-
Do not conduct electricity due to absence of free mobile ions or electrons.
Giant Covalent Molecules
-
High melting point: Strong covalent bonds hold the atoms together and require a lot of energy to overcome.
-
Unable to conduct electricity except graphite which contains delocalised electrons.
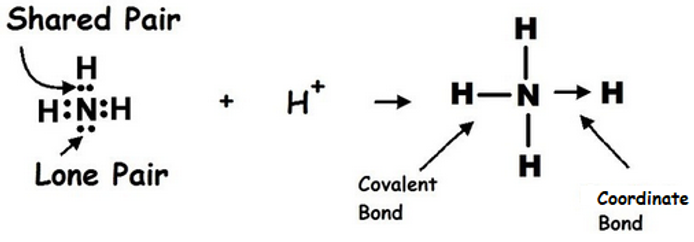
3.3.4 Dative Covalent Bonds
-
Dative covalent bonding is also known as coordinate bonding.
-
It occurs when the shared electrons come from the same atom.
-
The donor atom must have at least one pair of lone electrons.
-
Accepting atom must have vacant orbitals to accept the electrons.
3.4 Valence Shell Repulsion Theory
-
In molecules, electron pairs (bonding and lone) are arranged so as to maximise distance between election pairs, minimise electrostatic repulsion and maximise stability of molecules.
-
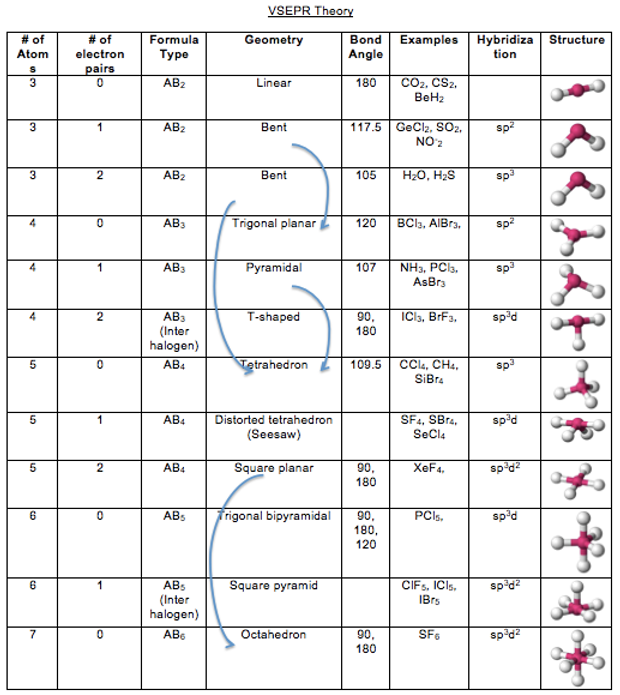
To predict shape, look at central atom and the number of lone and bonding electron pairs around it.
Making use of the VSEPR Theory
-
Draw out the Lewis structure for a molecule. (Include lone-pair and bonding pairs around the central atom) (Even double/triple bonds are considered a single bonding pair!)
-
Count the number of lone pair and bonding pair and make use of the table to identify the molecular shape and its bond angle.
-
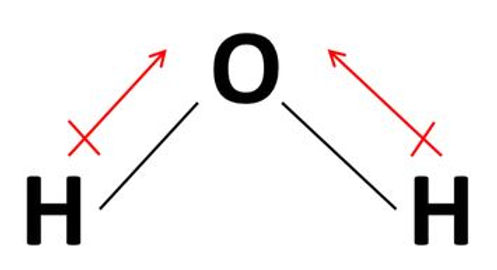
For example, in water, the central atom of oxygen has two lone pairs and two bonding pairs and hence by the table it is identified as a bent molecule with an angle of 105°.
Exceptions to the VSEPR Theory
-
Electronegativity of the central atom may affect actual bond angles. Greater electronegativities can result in larger bond angle. Higher electronegativities can result in greater pulling of bonding pairs towards itself causing increased repulsion between them which results in greater bond angles.
-
The number of lone pairs is another determinant of bond angle. Lone Pair-Lone Pair>Lone Pair-Bond Pair>Bond Pair-Bond Pair in terms of repulsion. Comparing CH4, NH3, H2O, one can note that there are 4 electron pairs but CH4 has 4 bonding pairs, NH3 has 3 bonding pairs and one lone pair, H2O has 2 bonding pairs and 2 lone pairs. The angle of H2O is 105°, NH3 is 107°, CH4 is 109.5° hence proving the earlier repulsion statement.
3.5 Electronegativity
-
It is the ability to attract bonding electrons to an atom.
-
It decreases down the group and increases across the period.
-
Most electronegative element is fluorine.
Ionic Character in Covalent Bonds
-
If there is an electronegativity difference between two atoms sharing electrons in a covalent bond, this bond may be attracted more by the more electronegative atom resulting in a permanent negative dipole towards that atom and hence a permanent positive dipole towards the other atom causing a polar bond. This results in an ionic character.
Covalent Character in Ionic Bonds
-
The cation may pull the electron cloud of the anion towards itself which can result in a covalent character.
-
This 'pull' is affected by the charge density of the cation and the anion.
-
The higher the charge density of the cation, the more it 'pulls' on the electron cloud of the anion causing a covalent character.
-
The lower the charge density of the anion, the more the anion is able to be 'polarised' easing the polarising effect of the cation.
-
A common example would be BeCl2 or AlCl3.
3.6 Polarity
-
In addition to what has been said under ionic character in covalent bonds, the entire molecule may be polar if it has a net dipole moment.
-
Water is polar as it a permanent net dipole acting upwards.
3.7 Intermolecular Forces of Attraction
Instantaneous dipole-induced dipole attraction
-
Electrons move randomly within a molecule which can result in a higher concentration of electrons at one side which can arise to a temporary net dipole in the molecule. This molecule then induces dipoles on neighbouring molecules. The opposite dipoles then attract arising to weak id-id bonds being formed.
-
It is affected by the size of the electron cloud. Higher the size, the more polarisable the electron cloud of the molecule and hence stronger id-id bonds.
-
It is also affected by surface area. Larger the surface area of the molecule the stronger the id-id interaction.
Permanent dipole-permanent dipole attraction
-
Stronger than id-id (not always!)
-
Forms when there is a net-dipole moment in the molecule. It is like earlier but it is not temporary.
-
Exists between polar molecules.
-
The more polar the molecule, the stronger the pd-pd interaction.
Hydrogen bonding
-
A more extreme case of pd-pd bonds.
-
If a hydrogen atom is attached to, Nitrogen, Oxygen or Fluorine atom which has a lone pair, hydrogen bonding will occur between the molecules.
-
The more hydrogen-lone pair sets the stronger the hydrogen bonds.
-
The more electronegative the element, the stronger the hydrogen bonds.
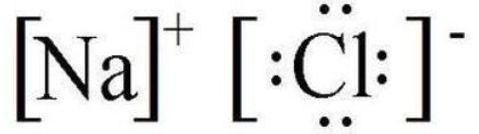



© 2018 Shanmugam Udhaya All Rights Reserved





